
Chemistry, 14.09.2019 07:10 Ididntwanttomakethis
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) what is the ph before any naoh is added? b) what is the ph after 5.00 ml of naoh are added? c) after 10 ml of naoh are added? d) what is the ph when 20 ml of naoh have been added? what is this point in the titration called?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1


Chemistry, 23.06.2019 12:30
All chemicals, even safe chemicals, such as sodium chloride( table salt), are toxic if exposure is high enough. (true or false)
Answers: 2
You know the right answer?
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) wha...
Questions

Computers and Technology, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50



History, 26.04.2021 06:50





Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50


Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

Mathematics, 26.04.2021 06:50

![\frac{[x][x] }{[0,200-x]}](/tpl/images/0231/0228/4f77c.png)
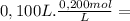 = 2,0x10⁻² mol
= 2,0x10⁻² mol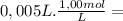 = 5,0x10⁻³ mol
= 5,0x10⁻³ mol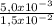
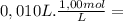 = 1,0x10⁻² mol
= 1,0x10⁻² mol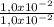
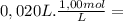 = 2,0x10⁻² mol
= 2,0x10⁻² mol![\frac{[x][x] }{[0,01667-x]}](/tpl/images/0231/0228/2af99.png)


