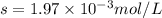Calcium sulfate is only sparingly soluble. caso4(s) ⇌ ca2+(aq) + so42-(aq) for this type of dissolution reaction the equilibrium constant, also known as the solubility product, is denoted ks. in the reaction above, ks = 3.9 x 10-6. when an excess of the solid is dissolved in water what is the maximum concentration of ca2+(aq) in mol l-1?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Calcium sulfate is only sparingly soluble. caso4(s) ⇌ ca2+(aq) + so42-(aq) for this type of dissolut...
Questions

Mathematics, 28.12.2019 00:31












Computers and Technology, 28.12.2019 00:31


Computers and Technology, 28.12.2019 00:31





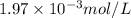
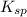
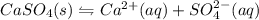
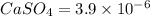
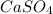 gives 1 mole of
gives 1 mole of 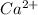 and 1 mole of
and 1 mole of 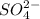 .
.![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0231/0233/958f2.png)
![3.9\times 10^{-6}=[s][s]](/tpl/images/0231/0233/47679.png)