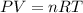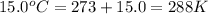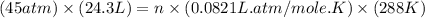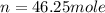Chemistry, 14.09.2019 07:10 littylai5524
Asample of neon gas collected at a pressure of l45 atm and a temperature of 15.0 c is found to occupy a velume of 24.3 liters how many moles of ne gas are in the sample mol submit answer retry entire group 2 more group attempts remaining

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Asample of neon gas collected at a pressure of l45 atm and a temperature of 15.0 c is found to occup...
Questions

Mathematics, 14.12.2020 06:50

Mathematics, 14.12.2020 06:50

Mathematics, 14.12.2020 06:50

Spanish, 14.12.2020 06:50


Geography, 14.12.2020 06:50

Mathematics, 14.12.2020 06:50


Mathematics, 14.12.2020 06:50



Health, 14.12.2020 06:50




Arts, 14.12.2020 06:50

Mathematics, 14.12.2020 06:50

Mathematics, 14.12.2020 06:50


Mathematics, 14.12.2020 06:50