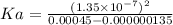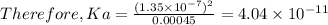Chemistry, 14.09.2019 07:30 cravens511peeelg
What is the ka of a weak acid (ha) if the initial concentration of weak acid is 4.5 x 10-4 m and the ph is 6.87? (pick one)
5.5 x 10-5
4.0 x 10-6
6.9 x 10-4
3.5 x 10-10
4.0 x 10-11

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
What is the ka of a weak acid (ha) if the initial concentration of weak acid is 4.5 x 10-4 m and the...
Questions



Mathematics, 25.02.2022 20:50


History, 25.02.2022 20:50



English, 25.02.2022 20:50

Social Studies, 25.02.2022 20:50



Chemistry, 25.02.2022 21:00

English, 25.02.2022 21:00





English, 25.02.2022 21:00


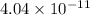
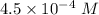
![pH = -log[H^+]](/tpl/images/0231/0476/7d119.png)
![[H^+]=10^{-pH}](/tpl/images/0231/0476/2646c.png)
![[H^+]=10^{-6.87}=1.35 \times 10^{-7}\ M](/tpl/images/0231/0476/ad497.png)
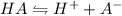
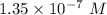
![Ka = \frac{[H^+][A^{-}]}{[HA]}](/tpl/images/0231/0476/9796e.png)