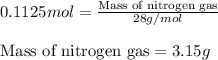Chemistry, 14.09.2019 07:30 qdogisbeast6132
For the following reaction, calculate how many grams of each product are formed when 4.05 g of water is used.
2 h20 > 2 h2 + o2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
For the following reaction, calculate how many grams of each product are formed when 4.05 g of water...
Questions


History, 07.12.2020 20:00

English, 07.12.2020 20:00


Social Studies, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00






History, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00


English, 07.12.2020 20:00

Physics, 07.12.2020 20:00

Biology, 07.12.2020 20:00

History, 07.12.2020 20:00

History, 07.12.2020 20:00

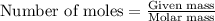 .....(1)
.....(1)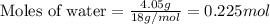
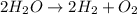
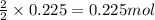 of hydrogen gas.
of hydrogen gas.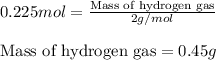
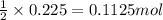 of nitrogen gas.
of nitrogen gas.