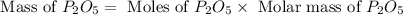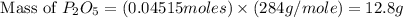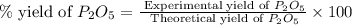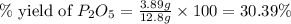Chemistry, 14.09.2019 07:30 holycow7868
Determine the percent yield of the following reaction when 2.80 g of p reacts with excess oxygen. the actual yield of this reaction is determined to by 3.89 g of p2o5.
4 p + 5 o2 > 2 p2o5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3
You know the right answer?
Determine the percent yield of the following reaction when 2.80 g of p reacts with excess oxygen. th...
Questions

Physics, 24.03.2021 17:40




Mathematics, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40

Biology, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

English, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Biology, 24.03.2021 17:40

English, 24.03.2021 17:40

English, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

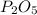 is, 30.39 %
is, 30.39 %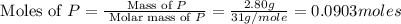
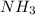
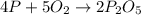
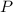 react to give 2 mole of
react to give 2 mole of 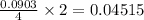 moles of
moles of