Calculate δs°for the combustion of ammonia.
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substanc...

Chemistry, 14.09.2019 08:20 serenityarts123
Calculate δs°for the combustion of ammonia.
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substance nh3(g) o2(g) n2(g) h2o(l)
s°(j/k·mol) 192 205.1 192 70
-135 j
-579 j
-387 j
579 j

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Questions

History, 19.04.2021 18:10

Mathematics, 19.04.2021 18:10

Mathematics, 19.04.2021 18:10

Mathematics, 19.04.2021 18:10

History, 19.04.2021 18:10


Social Studies, 19.04.2021 18:10


Mathematics, 19.04.2021 18:10





Social Studies, 19.04.2021 18:10






Chemistry, 19.04.2021 18:10

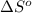 of the reaction is
of the reaction is 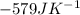
![\Delta S_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0231/1100/ec939.png)
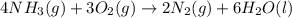
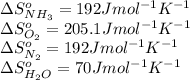
![\Delta S^o_{rxn}=[(6\times \Delta S^o_{H_2O})+(2\times \Delta S^o_{N_2})]-[(4\times \Delta S^o_{NH_3})+(3\times \Delta S^o_{O_2})]](/tpl/images/0231/1100/480ae.png)
![\Delta S^o_{rxn}=[(6\times 70)+(2\times 192)]-[(4\times 192)+(3\times 205.1)]=-579JK^{-1}](/tpl/images/0231/1100/8f98e.png)


