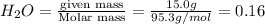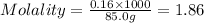Chemistry, 14.09.2019 08:30 trillralyn4060
Calculate the molality of a 150% by mass solution of mgcl, fw-95.3 g/mol in h. o. the density of tis solution is 1.127 gim 0.0134 m 0.157 m 1.58 m 1.86 m 11.8 m igator delete backspace u 10 pilli

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Calculate the molality of a 150% by mass solution of mgcl, fw-95.3 g/mol in h. o. the density of tis...
Questions



Advanced Placement (AP), 13.07.2019 22:40







Mathematics, 13.07.2019 22:50

English, 13.07.2019 22:50

Mathematics, 13.07.2019 22:50

Arts, 13.07.2019 22:50

English, 13.07.2019 22:50

History, 13.07.2019 22:50

History, 13.07.2019 22:50


Mathematics, 13.07.2019 22:50

Spanish, 13.07.2019 22:50

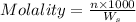
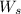 = weight of solvent in g
= weight of solvent in g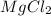 is present in 100 g of solution
is present in 100 g of solution