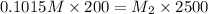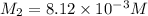Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
A200 ml sample of 0.1015 miric acid is mbred with 2300 ml of water. what is the molar concentration...
Questions

SAT, 16.04.2021 04:20





Mathematics, 16.04.2021 04:20






Mathematics, 16.04.2021 04:20



Mathematics, 16.04.2021 04:20




Mathematics, 16.04.2021 04:20

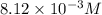
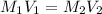
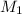 = molarity of stock
= molarity of stock 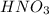 solution = 0.1015 M
solution = 0.1015 M
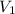 = volume of stock
= volume of stock 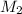 = molarity of dilute
= molarity of dilute 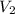 = volume of dilute
= volume of dilute