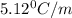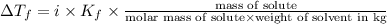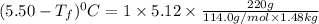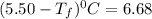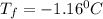Chemistry, 14.09.2019 08:30 lineaeriksen
Calculate the freezing point of a solution made from 220g of octane (c hua), molar mass = 114,0 gmol dissolved in 1480 g of benzene. benzene freezes at 5.50"c and its kvalue is 5.12c/m. -1.16°c 0.98°c 666"c 12 2°c 5.49°c 10 12 am a a 2019 backspace yuo pill но кl

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Calculate the freezing point of a solution made from 220g of octane (c hua), molar mass = 114,0 gmol...
Questions

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Geography, 26.03.2021 04:00

Biology, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Advanced Placement (AP), 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00


Mathematics, 26.03.2021 04:00




History, 26.03.2021 04:10

Mathematics, 26.03.2021 04:10

Mathematics, 26.03.2021 04:10

History, 26.03.2021 04:10

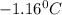
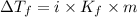
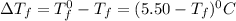 = Depression in freezing point
= Depression in freezing point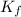 = freezing point constant =
= freezing point constant =