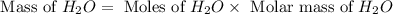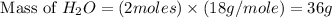Chemistry, 14.09.2019 08:30 anthonyhaywood
For the following reaction, 101 grams of magnesium nitride are allowed to react with 144 grams of water. mg3n2 (5) + 6 h20 (1) — 3 mg(oh)2 (aq) + 2 nh2 (aq) what is the formula for the limiting reagent? what is the maximum amount of magnesium hydroxide that can be formed? grams what amount of the excess reagent remains after the reaction is complete? grams submit answer retry entire group 8 more group attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
For the following reaction, 101 grams of magnesium nitride are allowed to react with 144 grams of wa...
Questions


Health, 22.01.2021 18:40




History, 22.01.2021 18:40


Mathematics, 22.01.2021 18:40




Mathematics, 22.01.2021 18:40





History, 22.01.2021 18:40

Health, 22.01.2021 18:40


History, 22.01.2021 18:40

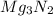 .
.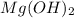 is, 174 grams.
is, 174 grams.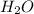 = 144 g
= 144 g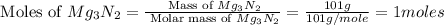
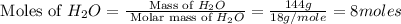
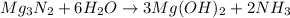
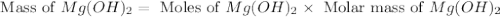
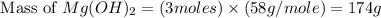
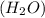 .
.