
Chemistry, 14.09.2019 08:30 webbjalia04
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane and gaseous water in a 0.379 l flask at 1191 k. at equilibrium, the flask contains 0.145 mol of co gas, 0.218 mol of h2 gas, and 0.25 mol of methane. what is the water concentration at equilibrium (kc = 0.30 for this process at 1191 k)?
enter to 4 decimal places.
hint: look at sample problem 17.7 in the 8th ed silberberg book. write a balanced chemical equation. write the kc expression. calculate the equilibrium concentrations of all the species given (moles/liter). put values into kc expression, solve for the unknown.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane an...
Questions



Social Studies, 19.10.2019 02:30


History, 19.10.2019 02:30

Mathematics, 19.10.2019 02:30



Mathematics, 19.10.2019 02:30

Mathematics, 19.10.2019 02:30




Chemistry, 19.10.2019 02:30

Mathematics, 19.10.2019 02:30



Mathematics, 19.10.2019 02:40

Physics, 19.10.2019 02:40

History, 19.10.2019 02:40

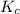
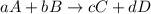
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0231/1245/b6f47.png)
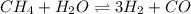
![K_{c}=\frac{[H_2]^3[CO]}{[CH_4][H_2O]}](/tpl/images/0231/1245/e7210.png) ....(1)
....(1)
![[CO]=\frac{0.145mol}{0.379L}=0.383mol/L](/tpl/images/0231/1245/315b1.png)
![[H_2]=\frac{0.218mol}{0.379L}=0.575mol/L](/tpl/images/0231/1245/b555b.png)
![[CH_4]=\frac{0.25mol}{0.379L}=0.660mol/L](/tpl/images/0231/1245/35995.png)

![0.30=\frac{(0.575)^3\times 0.383}{0.660\times [H_2O]}](/tpl/images/0231/1245/8e17e.png)
![[H_2O]=\frac{(0.575)^3\times 0.383}{0.660\times 0.30}=0.3677](/tpl/images/0231/1245/f4553.png)


