
Chemistry, 14.09.2019 08:30 Aurionna101
A.) how would you prepare .250 l of a 0.300 m phosphate buffer at ph=3 (h3po4: pka1 = 2.12, pka2 = 7.21, pka = 12.32) using the appropriate weak acid and conjugate salt. b.) how would you prepare the assigned buffer (given in question #1a) if using combining the weak acid and 0.400 m naoh? c.) how would you prepare the assigned buffer (given in question #1a) if using combining the weak acid and 0.400 m hcl?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1


Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
A.) how would you prepare .250 l of a 0.300 m phosphate buffer at ph=3 (h3po4: pka1 = 2.12, pka2 =...
Questions

Physics, 27.08.2019 05:40


Spanish, 27.08.2019 05:40


Chemistry, 27.08.2019 05:40




Social Studies, 27.08.2019 05:40


Business, 27.08.2019 05:40

Mathematics, 27.08.2019 05:40







Mathematics, 27.08.2019 05:40

Mathematics, 27.08.2019 05:40

![\frac{[H2PO4-]}{[H3PO4]}](/tpl/images/0231/1239/e366e.png)
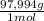 = 0,857 g of H₃PO₄
= 0,857 g of H₃PO₄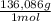 = 9,016 g of KH₂PO₄
= 9,016 g of KH₂PO₄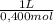 = 0,166 L ≡ 166 mL of 0,400M NaOH
= 0,166 L ≡ 166 mL of 0,400M NaOH

