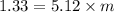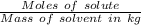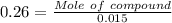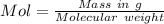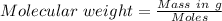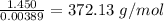A1.450 g sample of an unknown organic compound , x, is dissolved in 15.0 g of toluene
( c7h8 =...

Chemistry, 14.09.2019 09:10 allendm5166
A1.450 g sample of an unknown organic compound , x, is dissolved in 15.0 g of toluene
( c7h8 = 92 g/mol) and the freezing point is lowered by 1.33 oc. what is the molecular weight
of the organic compound? (kf = 5.12 oc/m).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Questions


Mathematics, 31.01.2022 05:30






Mathematics, 31.01.2022 05:40


Chemistry, 31.01.2022 05:40

Mathematics, 31.01.2022 05:40



Mathematics, 31.01.2022 05:40





Mathematics, 31.01.2022 05:40

History, 31.01.2022 05:40

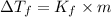
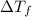 = Depression in freezing point
= Depression in freezing point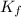 = Molal depression constant
= Molal depression constant