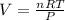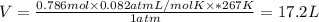Areaction at -6.0 °c evolves 786. mmol of sulfur tetrafluoride gas. calculate the volume of sulfur tetrafluoride gas that is collected. you can assume the pressure in the room is exactly 1 atm. be sure your answer has the correct number of significant digits. volume: 1 x i

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Areaction at -6.0 °c evolves 786. mmol of sulfur tetrafluoride gas. calculate the volume of sulfur t...
Questions

Medicine, 06.08.2021 22:10

Mathematics, 06.08.2021 22:10




Spanish, 06.08.2021 22:20

Chemistry, 06.08.2021 22:20

Arts, 06.08.2021 22:20

English, 06.08.2021 22:20

Mathematics, 06.08.2021 22:20

Mathematics, 06.08.2021 22:20

Mathematics, 06.08.2021 22:20

Computers and Technology, 06.08.2021 22:20