
Chemistry, 14.09.2019 09:30 babygurl27732
If 1.0 m hcl is added to an equal volume of 0.2 m naoh, what are the new concentrations of oh and h30+ ? select one: [h30*] = 0.8 m, [oh]= 1.3 x 10-14 m h30*] = 0.5 m, [oh]= 0.1 mi h30*] = 0.4 m, [oh]= 2.5 x 1014 m h30*] 1.0 m, [0h]= 0.2 m h30*] = 0.5 m, [oh]= 2.0 x 1014 m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

You know the right answer?
If 1.0 m hcl is added to an equal volume of 0.2 m naoh, what are the new concentrations of oh and h3...
Questions


Social Studies, 29.08.2019 13:00


Chemistry, 29.08.2019 13:00


Mathematics, 29.08.2019 13:00

Mathematics, 29.08.2019 13:00

History, 29.08.2019 13:00








Computers and Technology, 29.08.2019 13:00




Social Studies, 29.08.2019 13:00

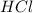 is a strong acid and
is a strong acid and 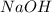 is a strong base. The resulting reaction is a neutralization reaction forming
is a strong base. The resulting reaction is a neutralization reaction forming 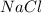 and
and 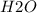

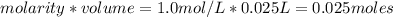
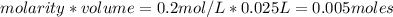
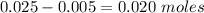
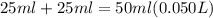
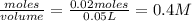
![[H3O+][OH-] = 10^{-14}\\ \\[OH-] = \frac{10^{-14} }{[H3O+]} = \frac{10^{-14} }{0.4} =2.5*10^{-14}](/tpl/images/0231/2255/824f2.png)


