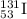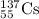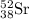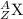Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Write the isotopic symbol for the following (show your work) a) an isotope of iodine whose atoms hav...
Questions

Mathematics, 02.10.2020 23:01

Health, 02.10.2020 23:01


History, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01


Mathematics, 02.10.2020 23:01

Physics, 02.10.2020 23:01

Spanish, 02.10.2020 23:01

Social Studies, 02.10.2020 23:01

Social Studies, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01





Mathematics, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01