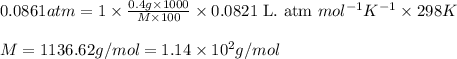Chemistry, 14.09.2019 10:10 magicalpenguin48
400. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmotic pressure of this solution is measured to be 0.0861 atm at 25.0 °c. calculate the molar mass of the protein. round your answer to 3 significant digits. mol x 6 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1


Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
400. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmot...
Questions





English, 15.12.2019 03:31






English, 15.12.2019 03:31

Biology, 15.12.2019 03:31

Mathematics, 15.12.2019 03:31

Biology, 15.12.2019 03:31


Mathematics, 15.12.2019 03:31


Mathematics, 15.12.2019 03:31


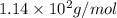
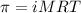
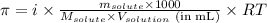
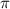 = Osmotic pressure of the solution = 0.0861 atm
= Osmotic pressure of the solution = 0.0861 atm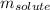 = mass of protein = 400 mg = 0.4 g (Conversion factor: 1 g = 1000 mg)
= mass of protein = 400 mg = 0.4 g (Conversion factor: 1 g = 1000 mg)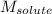 = molar mass of protein = ?
= molar mass of protein = ?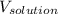 = Volume of solution = 5.00 mL
= Volume of solution = 5.00 mL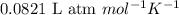
![25^oC=[25+273]K=298K](/tpl/images/0231/2487/df1f6.png)