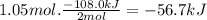Chemistry, 14.09.2019 10:20 coontcakes
the decomposition of hydrogen peroxide, h2o2, has been used to provide thrust in the control jets of various space vehicles. using the supplemental data, determine how much heat (in kj) is produced by the decomposition of 1.05 mol of h2o2 under standard conditions.
2 h2o2(l) → 2 h2o(g) + o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
the decomposition of hydrogen peroxide, h2o2, has been used to provide thrust in the control jets of...
Questions





Computers and Technology, 06.03.2020 20:02

Mathematics, 06.03.2020 20:03

English, 06.03.2020 20:03




Computers and Technology, 06.03.2020 20:03









Mathematics, 06.03.2020 20:04