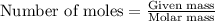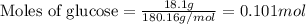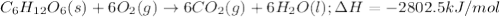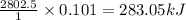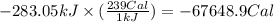Chemistry, 14.09.2019 10:20 GalaxyCraft4991
the oxidation of the sugar glucose, c6h12o6, is described by the following equation. c6h12o6(s) + 6 o2(g) → 6 co2(g) + 6 h2o(l) δh = −2802.5 kj/mol the metabolism of glucose gives the same products, although the glucose reacts with oxygen in a series of steps in the body.
(a) how much heat in kilojoules can be produced by the metabolism of 18.1 g of glucose?
(b) how many calories can be produced by the metabolism of 18.1 g of glucose?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
the oxidation of the sugar glucose, c6h12o6, is described by the following equation. c6h12o6(s) + 6...
Questions

Mathematics, 30.10.2020 15:30

Mathematics, 30.10.2020 15:30

Mathematics, 30.10.2020 15:30


Mathematics, 30.10.2020 15:30

English, 30.10.2020 15:30







History, 30.10.2020 15:30



Mathematics, 30.10.2020 15:30


Chemistry, 30.10.2020 15:30

Mathematics, 30.10.2020 15:30

Mathematics, 30.10.2020 15:30