
Chemistry, 14.09.2019 10:30 tristanortonubca
Nault 25000l 250 our = 25000l 15040 8. how many grams of cacl2 are needed to make 150.0 ml of a 0.500 m cf solution? (note: cacl2 is a soluble salt. the molar mass of cacl2 is 110.98 g/mol.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2
You know the right answer?
Nault 25000l 250 our = 25000l 15040 8. how many grams of cacl2 are needed to make 150.0 ml of a 0.50...
Questions


Physics, 11.10.2019 21:00

History, 11.10.2019 21:00







Chemistry, 11.10.2019 21:00





Arts, 11.10.2019 21:00

Mathematics, 11.10.2019 21:00



Mathematics, 11.10.2019 21:00

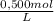 = 0,075 CaCl₂ moles
= 0,075 CaCl₂ moles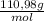 = 8,324 g of CaCl₂
= 8,324 g of CaCl₂

