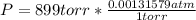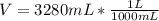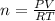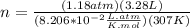part a;
the equation for molarity, m, is
m=n/v
where v is volume and n is the number of moles of solute.
a certain solution has a molarity of m = 2.73 mol/l and has a volume of v = 0.650 l . what is the value of n?
express your answer numerically in moles.
part b;
the equation for photon energy, e, is
e=hcλ
where h = 6.626×10−34 j⋅s (planck's constant) and c = 2.99×108 m/s (the speed of light).
what is the wavelength, λ, of a photon that has an energy of e = 3.98×10−19 j ?
express your answer numerically in meters.
part c;
the ideal gas equation is
pv=nrt
where p is pressure, v is volume, n is the number of moles, r is a constant, and t is temperature.
you are told that a sample of gas has a pressure of p = 899 torr , a volume of v = 3280 ml, and a temperature of t = 307 k . if you use r = 8.206×10−2 l⋅atm/(k⋅mol) , which of the following conversions would be necessary before you could find the number of moles of gas, n, in this sample?
check all that apply.
view available hint(s)
check all that apply.
convert the pressure to atmospheres (atm).
convert the pressure to pascals (pa).
convert the volume to cubic meters (m3).
convert the volume to liters (l).
convert the temperature to degrees celsius (∘c).
convert the temperature to degrees fahrenheit (∘f).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
part a;
the equation for molarity, m, is
m=n/v
where v is volume and n is the num...
the equation for molarity, m, is
m=n/v
where v is volume and n is the num...
Questions















Computers and Technology, 09.08.2021 20:30



Mathematics, 09.08.2021 20:30

Computers and Technology, 09.08.2021 20:30

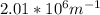
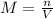
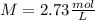 and the volume V = 0.650L, so you need to solve the equation for n:
and the volume V = 0.650L, so you need to solve the equation for n: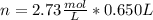
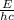
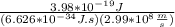
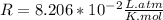 so you need to convert the pressure to atmospheres and convert the volume to liters.
so you need to convert the pressure to atmospheres and convert the volume to liters.