
Chemistry, 14.09.2019 11:10 averyiodonnell
Consider these compounds:
a. pbf2
b. ag2so3
c. nico3
d. agcl
complete the following statements by entering the letter(s) corresponding to the correct compound(s). (if more than one compound fits the description, include all the relevant compounds by writing your answer as a string of characters without punctuation, e. g, abc.)
without doing any calculations it is possible to determine that silver chromate is more soluble , and silver chromate is less soluble .
it is not possible to determine whether silver chromate is more or less soluble by simply comparing kspvalues.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
Consider these compounds:
a. pbf2
b. ag2so3
c. nico3
d. agcl
complete...
a. pbf2
b. ag2so3
c. nico3
d. agcl
complete...
Questions








Mathematics, 28.06.2020 22:01

Mathematics, 28.06.2020 22:01


Mathematics, 28.06.2020 22:01


Mathematics, 28.06.2020 22:01


Physics, 28.06.2020 22:01

History, 28.06.2020 22:01


Social Studies, 28.06.2020 22:01


![A_{n}B_{m} - nA^{m+}+mB^{n-} \\K_{s}=[A^{m}]^{n}[B^{n}]^{m}](/tpl/images/0231/3187/80c03.png)
![Ag_{2}CrO_{4}(s)-2Ag^{+}(aq)+CrO_{4} ^{2-}\\ s -2s+s\\s-[2s]^{2}[s]=4s^{3} \\\\A) PbF_{2}(s)-Pb^{2+} (aq)+2F^{-}\\s-s+2s\\s-[s][2s]^{2}=4s^{3}\\\\\\B)Ag_{2}SO_{3}(s)-2Ag^{+}(aq)+SO_{3} ^{2-}\\ s -2s+s\\s-[2s]^{2}[s]=4s^{3} \\\\\\C)NiCO_{3}(s)-Ni^{2+}(aq)+CO_{3} ^{2-}\\ s -s+s\\s-[s][s]=s^{2} \\\\\\D)AgCl(s)-Ag^{+}(aq)+Cl^{-}(aq)\\ s -s+s\\s-[s][s]=s^{2} \\](/tpl/images/0231/3187/955dd.png)
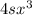 , same as the compound in options A and B, comparing these numbers you can't determine which one is bigger. Finally, options C and D have a kps of
, same as the compound in options A and B, comparing these numbers you can't determine which one is bigger. Finally, options C and D have a kps of 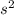 , this value is smaller than silver chromate's kps.
, this value is smaller than silver chromate's kps.

