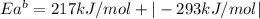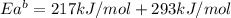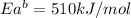Chemistry, 14.09.2019 11:10 joelpimentel
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and the change in enthalpy for the reaction is δh = -293 kj/mol .
what is the activation energy for the reverse reaction?
enter your answer numerically and in terms of kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and th...
Questions

Business, 27.09.2021 19:20




Mathematics, 27.09.2021 19:20


Mathematics, 27.09.2021 19:30

Social Studies, 27.09.2021 19:30

Mathematics, 27.09.2021 19:30



Chemistry, 27.09.2021 19:30


Computers and Technology, 27.09.2021 19:30

Mathematics, 27.09.2021 19:30





Mathematics, 27.09.2021 19:30

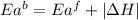
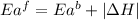
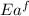 = activation energy for forward reaction
= activation energy for forward reaction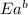 = activation energy for backward reaction
= activation energy for backward reaction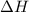 = change in enthalpy of reaction
= change in enthalpy of reaction