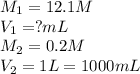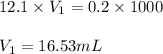Chemistry, 14.09.2019 11:20 maskythegamer
How many ml of concentrated hydrochloric acid would be required to make 1 l of a 0.2 m solution? assume that concentrated hydrochloric acid is 12.1 m

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
How many ml of concentrated hydrochloric acid would be required to make 1 l of a 0.2 m solution? as...
Questions



Health, 05.12.2019 15:31



History, 05.12.2019 15:31

English, 05.12.2019 15:31

Chemistry, 05.12.2019 15:31


History, 05.12.2019 15:31








Mathematics, 05.12.2019 15:31


English, 05.12.2019 15:31

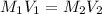
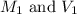 are the molarity and volume of the concentrated solution
are the molarity and volume of the concentrated solution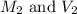 are the molarity and volume of diluted solution
are the molarity and volume of diluted solution