

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2


Chemistry, 23.06.2019 14:30
When does phenolphthalein turn pink? in the presence of a base in the presence of an acid when it is in a neutral solution when it is reacting with a metal
Answers: 1
You know the right answer?
Calculate the ph of: (a) 0.1m hcl; (b) 0.1m naoh; (c) 3 x 10% m hno3; (d) 5 x 10-10 m hcio.; an...
Questions



History, 23.09.2020 05:01

Mathematics, 23.09.2020 05:01

Mathematics, 23.09.2020 05:01

Mathematics, 23.09.2020 05:01

Mathematics, 23.09.2020 05:01

Mathematics, 23.09.2020 05:01

Mathematics, 23.09.2020 05:01






Mathematics, 23.09.2020 05:01


Computers and Technology, 23.09.2020 05:01



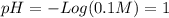
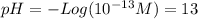
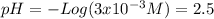
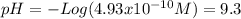
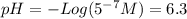
![pH = -Log ([H^{+}]) = 1](/tpl/images/0231/3444/7f8ff.png)
![K_{w} =[H^{+} ][OH^{-}]=10^{-14}](/tpl/images/0231/3444/e0923.png)
![[H^{+} ]](/tpl/images/0231/3444/cd271.png)
![[H^{+} ]=\frac{10^{-14}}{[OH^{-}]}](/tpl/images/0231/3444/6c18c.png)
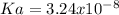
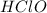 →
→ 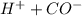 so the equilibrium is
so the equilibrium is 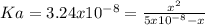
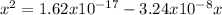
![x=[H^{+}]=4.93x10^{-10}](/tpl/images/0231/3444/c1c55.png)


