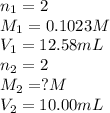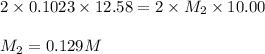Chemistry, 14.09.2019 11:30 chloehall2269
Ranjit titrates a sample 10.00 ml of ba(oh)2 solution to the endpoint using 12.58 ml of 0.1023 m h2so4.
based on this data, calculate the concentration of the barium hydroxide solution.
[ba(oh)2] = m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Ranjit titrates a sample 10.00 ml of ba(oh)2 solution to the endpoint using 12.58 ml of 0.1023 m h2s...
Questions

History, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00



Chemistry, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00


Mathematics, 05.12.2020 01:00

History, 05.12.2020 01:00





Mathematics, 05.12.2020 01:00



Mathematics, 05.12.2020 01:00



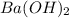 comes out to be 0.129 M.
comes out to be 0.129 M.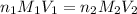
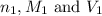 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 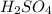
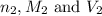 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is