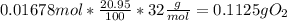Chemistry, 14.09.2019 11:30 loredobrooke9929
At 14,000 ft elevation the air pressure drops to 0.59 atm. assume you take a 1l sample of air at this altitude and compare it to 1 l of air taken at sea level. how much less o2 (in g) is available in 1 l of air at 14,000 ft (assume temperature of 298 k and that relative gas percentages are constant in both locations).

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
At 14,000 ft elevation the air pressure drops to 0.59 atm. assume you take a 1l sample of air at thi...
Questions



Mathematics, 17.07.2020 22:01


Mathematics, 17.07.2020 22:01







Mathematics, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01






Mathematics, 17.07.2020 22:01