

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
When carbon is burned in air, it reacts with oxygen to form carbon dioxide. when 20.4 g of carbon we...
Questions

Mathematics, 05.03.2021 05:20



Biology, 05.03.2021 05:20



Mathematics, 05.03.2021 05:20




Social Studies, 05.03.2021 05:20

English, 05.03.2021 05:20



Mathematics, 05.03.2021 05:20


Mathematics, 05.03.2021 05:20

Mathematics, 05.03.2021 05:20

History, 05.03.2021 05:20

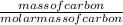
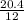 = 1.7moles
= 1.7moles

