
Chemistry, 16.09.2019 17:10 briweaver9993
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2co the reaction of 7.39 kg titanium dioxide with excess c and cl₂ gives 14.24 kg titanium tetrachloride. calculate the theoretical yield of ticl₄ (assuming complete reaction) and its percentage yield.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1


Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2...
Questions

Mathematics, 18.11.2019 00:31




History, 18.11.2019 00:31


Computers and Technology, 18.11.2019 00:31




Chemistry, 18.11.2019 00:31


English, 18.11.2019 00:31


Mathematics, 18.11.2019 00:31




Mathematics, 18.11.2019 00:31

History, 18.11.2019 00:31

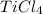 and its percentage yield is 81.0%
and its percentage yield is 81.0%
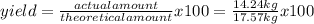 =81.0%
=81.0%

