
Phosphorus forms several compounds with oxygen, including diphosphorus trioxide and diphosphorus pentoxide. a decomposition of a sample of diphosphorus trioxide forms 1.29 g phosphorus to every 1.00 g oxygen. the decomposition of a sample of diphosphorus pentoxide forms 0.775 g phosphorus to every 1.00 g oxygen. show that these results are consistent with the law of multiple proportions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 13:00
Johnny's bakery has 30,900 grams of sugar. a recipe calls for 32 pounds of sugar to be used. how much sugar will be left over? (1 lb=453.59 g).
Answers: 2
You know the right answer?
Phosphorus forms several compounds with oxygen, including diphosphorus trioxide and diphosphorus pen...
Questions


Health, 12.11.2020 21:50

Biology, 12.11.2020 21:50

English, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50


Chemistry, 12.11.2020 21:50

Chemistry, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50







Mathematics, 12.11.2020 21:50

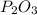 the ratio between P and O is
the ratio between P and O is 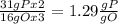 , that is consistent with the experimental result.
, that is consistent with the experimental result.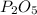 the ratio between P and O is
the ratio between P and O is 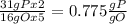 that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.
that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.


