
Chemistry, 16.09.2019 22:00 ericahale3971
Suppose 6.54g of potassium bromide is dissolved in 50.ml of a 0.70 m aqueous solution of silver nitrate. calculate the final molarity of bromide anion in the solution. you can assume the volume of the solution doesn't change when the potassium bromide is dissolved in it.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Suppose 6.54g of potassium bromide is dissolved in 50.ml of a 0.70 m aqueous solution of silver nitr...
Questions


Mathematics, 14.11.2019 09:31





History, 14.11.2019 09:31

Mathematics, 14.11.2019 09:31





English, 14.11.2019 09:31

English, 14.11.2019 09:31



Mathematics, 14.11.2019 09:31

Mathematics, 14.11.2019 09:31

English, 14.11.2019 09:31

History, 14.11.2019 09:31

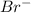 ]=0.4M
]=0.4M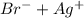 ⇄
⇄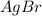 Kps for this reaction is
Kps for this reaction is 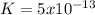 , this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.
, this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.![[Br^{-}]=6.54gKBr.\frac{1molKBr}{119gKBr}.\frac{1molBr^{-} }{1molKBr} .\frac{1}{0.05L} =1.10M](/tpl/images/0234/3240/bfec1.png)
![Qps=[Br^{-}][Ag^{+}]](/tpl/images/0234/3240/78356.png) =
=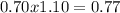
![[Br^{-}]=\frac{(\frac{1.1mol}{L}.0.05L-\frac{0.70mol}{L}.0.05L}{0.05L} =0.4M](/tpl/images/0234/3240/ff056.png)


