
Chemistry, 17.09.2019 00:10 jewlbug4358
Question 9 suppose 15.3g of sodium bromide is dissolved in 250.ml of a 0.40 m aqueous solution of silver nitrate. calculate the final molarity of bromide anion in the solution. you can assume the volume of the solution doesn't change when the sodium bromide is dissolved in it.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

You know the right answer?
Question 9 suppose 15.3g of sodium bromide is dissolved in 250.ml of a 0.40 m aqueous solution of si...
Questions

Chemistry, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01


Computers and Technology, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01


English, 19.09.2020 01:01


History, 19.09.2020 01:01










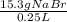 ×
× 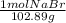 =
= 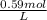 = 0.59 M
= 0.59 M

