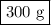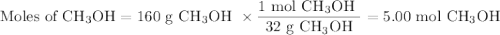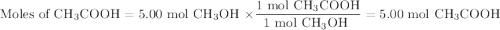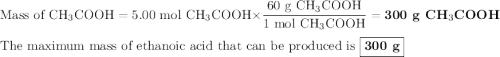The following reaction produces ethanoic acid (chacooh) from methanol (ch3oh) and carbon
monox...

Chemistry, 17.09.2019 01:00 ayeelol1447
The following reaction produces ethanoic acid (chacooh) from methanol (ch3oh) and carbon
monoxide
chcooh
ch3oh + co
relative atomie mass: h = 1: 0 = 16; c = 12
calculate the maximum mass of ethanoic acid that can be produced from 160g methanol,
assuming the carbon monoxide is in excess.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
Questions










Mathematics, 04.05.2021 03:40

Mathematics, 04.05.2021 03:40




Mathematics, 04.05.2021 03:40


Mathematics, 04.05.2021 03:40



Mathematics, 04.05.2021 03:40