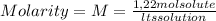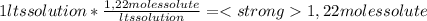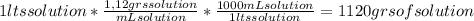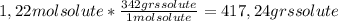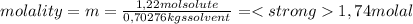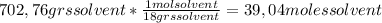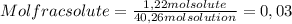Chemistry, 17.09.2019 02:00 romaguera06
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the solution is 1.12 g/ml. b) what is the mass percent of sucrose in this solution? c) what is the mole fraction of sucrose in this solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the...
Questions


History, 25.04.2022 23:00

Mathematics, 25.04.2022 23:00



Computers and Technology, 25.04.2022 23:10




SAT, 25.04.2022 23:10




Mathematics, 25.04.2022 23:20



Spanish, 25.04.2022 23:40



Chemistry, 25.04.2022 23:40