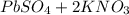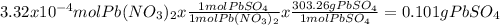Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
When aqueous solutions of k₂so₄ and pb(no₃)₂ are combined, pbso₄ precipitates. calculate the mass, i...
Questions


Social Studies, 09.07.2019 18:40

Spanish, 09.07.2019 18:40

Mathematics, 09.07.2019 18:40


Chemistry, 09.07.2019 18:40

Computers and Technology, 09.07.2019 18:40

SAT, 09.07.2019 18:40

Geography, 09.07.2019 18:40


English, 09.07.2019 18:50

English, 09.07.2019 18:50



Mathematics, 09.07.2019 18:50

Computers and Technology, 09.07.2019 18:50

Mathematics, 09.07.2019 18:50

SAT, 09.07.2019 18:50


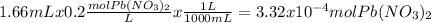
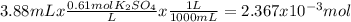
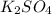
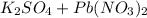 ⇒
⇒