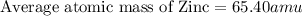2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu; 27.90% of 66zn with an atomic weight of 65.926 amu; 4.10% of 67zn with an atomic weight of 66.927 amu; 18.75% of 68zn with an atomic weight of 67.925 amu; and 0.62% of 70zn with an atomic weight of 69.925 amu. calculate the average atomic weight of zn.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

You know the right answer?
2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu...
Questions

Mathematics, 07.07.2019 02:30






Mathematics, 07.07.2019 02:30

Biology, 07.07.2019 02:30

History, 07.07.2019 02:30

Physics, 07.07.2019 02:30

History, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30

Advanced Placement (AP), 07.07.2019 02:30

History, 07.07.2019 02:30


Business, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30

Social Studies, 07.07.2019 02:30


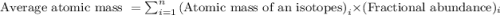 .....(1)
.....(1)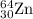 isotope:
isotope: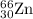 isotope:
isotope: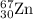 isotope:
isotope: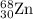 isotope:
isotope: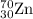 isotope:
isotope:![\text{Average atomic mass of Zinc}=[(63.929\times 0.4863)+(65.926\times 0.2790)+(66.927\times 0.0410)+(67.925\times 0.1875)+(69.925\times 0.0062)]](/tpl/images/0237/0119/27e73.png)