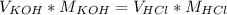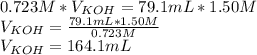Chemistry, 17.09.2019 19:00 chaseking120418
Titration is a type of experiment that can be performed to investigate a neutralization reaction. the equivalence point is when all of the acid and base is fully neutralized. a sample of 0.723 m aqueous potassium hydroxide was titrated against a standard solution of hydrochloric acid. what was the volume of the potassium hydroxide solution if 79.1 ml of 1.50 m hydrochloric acid was needed to reach the equivalence point?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Titration is a type of experiment that can be performed to investigate a neutralization reaction. th...
Questions


Mathematics, 25.07.2021 21:50



Mathematics, 25.07.2021 21:50

History, 25.07.2021 21:50


Chemistry, 25.07.2021 21:50





Chemistry, 25.07.2021 21:50


Mathematics, 25.07.2021 21:50

English, 25.07.2021 21:50

Mathematics, 25.07.2021 21:50


English, 25.07.2021 21:50