
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is mixed with 11.5 l of o2(g) at 2.66 atm and 298 k.(a) calculate the number of moles of each of the following before the reaction occurs.(i) fe(s)(ii) o2(g)(b) identify the limiting reactant when the mixture is heated to produce fe2o3. support youranswer with calculations.(c) calculate the number of moles of fe2o3 produced when the reaction proceeds tocompletion.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is...
Questions




Mathematics, 09.11.2020 22:10

Computers and Technology, 09.11.2020 22:10

History, 09.11.2020 22:10

Biology, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10




Mathematics, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10

Biology, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10

Mathematics, 09.11.2020 22:10


Mathematics, 09.11.2020 22:10

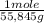 = 1,34 Fe(s) moles
= 1,34 Fe(s) moles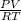 = n
= n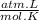
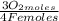 = 1,01 O₂ moles
= 1,01 O₂ moles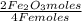 =
= 

