
Based on their activation energies and energy changes and assuming that all collision factors are the same, which of the following reactions would be fastest? (a) ea = 57 kj/mol , δe = 13 kj/mol (b) ea = 47 kj/mol , δe = -29 kj/mol (c) ea = 34 kj/mol , δe = -10 kj/mol .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3


Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

You know the right answer?
Based on their activation energies and energy changes and assuming that all collision factors are th...
Questions

Mathematics, 04.12.2020 20:30

History, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30



Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

English, 04.12.2020 20:30


Mathematics, 04.12.2020 20:30

History, 04.12.2020 20:30



Social Studies, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30


Mathematics, 04.12.2020 20:30

English, 04.12.2020 20:30

![r=k.[A]^{n}](/tpl/images/0237/1684/d8a47.png) [1]
[1]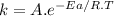 [2]
[2]

