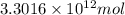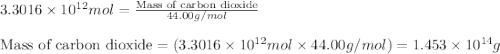Chemistry, 17.09.2019 20:10 samanthasheets8006
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (in grams) of co2 produced by the combustion of 1.80 x 1010 gallons of gasoline (the estimated annual consumption of gasoline in the u. remember, there are 3.785 liters in 1 gallon and assume that isooctane is the only carbon containing component of gasoline. scientific notation can be entered as follows: 1.23 x 1023 = 1.23e23

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (i...
Questions


Physics, 09.10.2019 17:00

History, 09.10.2019 17:00

Mathematics, 09.10.2019 17:00


History, 09.10.2019 17:00



World Languages, 09.10.2019 17:00

Physics, 09.10.2019 17:00


Mathematics, 09.10.2019 17:00




Mathematics, 09.10.2019 17:00

English, 09.10.2019 17:00

Chemistry, 09.10.2019 17:00

English, 09.10.2019 17:00

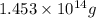
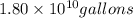
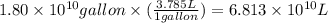
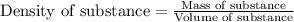
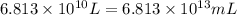 (Conversion factor: 1 L = 1000 mL)
(Conversion factor: 1 L = 1000 mL)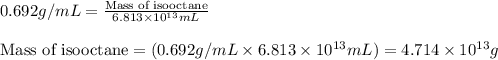
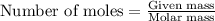 .....(1)
.....(1)
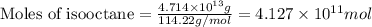
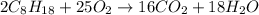
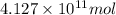 of isooctane will produce =
of isooctane will produce = 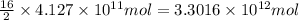 of carbon dioxide
of carbon dioxide