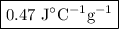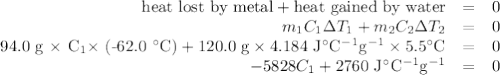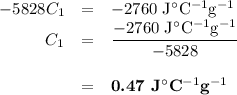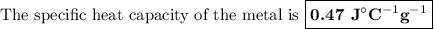A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the final temperature in the calorimeter is 32.0 °c. determine the specific heat of the metal. show your work by listing various steps, and explain how the law of conservation of energy applies to this situation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

You know the right answer?
A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the...
Questions


Computers and Technology, 27.12.2020 14:00

Mathematics, 27.12.2020 14:00


Mathematics, 27.12.2020 14:00

English, 27.12.2020 14:00




Mathematics, 27.12.2020 14:00

Biology, 27.12.2020 14:00

Mathematics, 27.12.2020 14:00

Mathematics, 27.12.2020 14:00



Biology, 27.12.2020 14:00



Mathematics, 27.12.2020 14:00