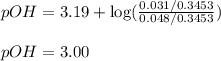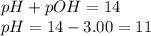Chemistry, 17.09.2019 21:00 ramenbates81
An analytical chemist is titrating 220.3 ml of 0.36 m solution of ethylamine with a 0.26 m hno3. calculate the ph of the base solution after the chemist has added 125 ml of the hno3 solution to it.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1
You know the right answer?
An analytical chemist is titrating 220.3 ml of 0.36 m solution of ethylamine with a 0.26 m hno3. cal...
Questions



Arts, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10



Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

History, 01.06.2021 20:10

English, 01.06.2021 20:10


Mathematics, 01.06.2021 20:10


Mathematics, 01.06.2021 20:10


Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

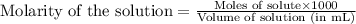
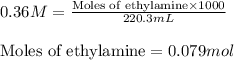

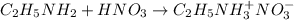
![pOH=pK_b+\log(\frac{[salt]}{[base]})](/tpl/images/0237/4463/db86b.png)
![pOH=pK_b+\log(\frac{[C_2H_5NH_3^+NO_3^-]}{[C_2H_5NH_2]})](/tpl/images/0237/4463/880ef.png)
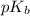 = negative logarithm of base dissociation constant of ethylamine = 3.19
= negative logarithm of base dissociation constant of ethylamine = 3.19![[C_2H_5NH_3^+NO_3^-]=\frac{0.031}{0.3453}](/tpl/images/0237/4463/701b1.png)
![[C_2H_5NH_2]=\frac{0.048}{0.3453}](/tpl/images/0237/4463/25ef0.png)