
Chemistry, 17.09.2019 21:00 ashvinmsingh
Achemist needs to create a series of standard cu2+(aq)cu2+(aq) solutions for an absorbance experiment. for the first standard, he uses a pipet to transfer 5.005.00 ml of a 2.172.17 m cu2+(aq)cu2+(aq) stock solution to a 500.0500.0 ml volumetric flask, and he adds enough water to dilute to the mark. he then uses a second pipet to transfer 25.0025.00 ml of the second solution to a 100.0100.0 ml volumetric flask, and he adds enough water to dilute to the mark. calculate the concentration of the cu2+(aq)cu2+(aq) solution in the 100.0100.0 ml volumetric flask.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Achemist needs to create a series of standard cu2+(aq)cu2+(aq) solutions for an absorbance experimen...
Questions

Biology, 03.11.2020 22:00

Mathematics, 03.11.2020 22:00

Business, 03.11.2020 22:00







History, 03.11.2020 22:00

English, 03.11.2020 22:00

Physics, 03.11.2020 22:00


Chemistry, 03.11.2020 22:00



History, 03.11.2020 22:00

Mathematics, 03.11.2020 22:00

Mathematics, 03.11.2020 22:00

Mathematics, 03.11.2020 22:00

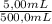 = 0,0217 M Cu²⁺solution
= 0,0217 M Cu²⁺solution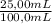 = 5,425×10⁻³ M Cu²⁺ solution
= 5,425×10⁻³ M Cu²⁺ solution

