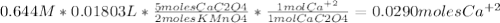Chemistry, 18.09.2019 00:00 EwTrTw4584
Calcium ion (ca2+) is necessary for the clotting of blood and many other physiological processes. a particular alien race has similar physiological processes except their concentration of ca2+ in blood is much higher than in humans. to measure the ca2+ concentration in 1.00 ml of alien blood, sodium oxalate (na2c2o4) solution is added, which precipitates the ca2+ as cac2o4. this solid is dissolved in dilute sulfuric acid to release c2o42latex: -−, and 18.03 ml of 0.644 m potassium permanganate is required to reach the end point. the balanced equation is 2kmno4(aq) + 5cac2o4(s) + 8 h2so4(aq) latex: \longrightarrow⟶ 2mnso4 (aq) + k2so4(aq) + 5caso4(s) +10 co2(g) +8h2o(l) calculate the amount (in mol) of ca2+ in 1.00 ml of alien blood. enter to 4 decimal places

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:00
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely.analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
Calcium ion (ca2+) is necessary for the clotting of blood and many other physiological processes. a...
Questions

Biology, 14.11.2019 18:31



History, 14.11.2019 18:31





Chemistry, 14.11.2019 18:31


History, 14.11.2019 18:31







Computers and Technology, 14.11.2019 18:31