
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is at a total pressure of 150 kpa, and a temperature of 500 k. the mixture has the following composition in mole fraction: 0.25 helium, 0.25 argon, 0.25 krypton. determine: (a) the mass fraction of helium. (b) the average molecular weight of the mixture. (c) the total molar concentration. (d) the mass density.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is a...
Questions

Mathematics, 25.09.2019 15:00

World Languages, 25.09.2019 15:00

Mathematics, 25.09.2019 15:00

Mathematics, 25.09.2019 15:00

Mathematics, 25.09.2019 15:00



Biology, 25.09.2019 15:00






Health, 25.09.2019 15:00

Mathematics, 25.09.2019 15:00


History, 25.09.2019 15:00

Social Studies, 25.09.2019 15:00

History, 25.09.2019 15:00

English, 25.09.2019 15:00

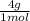 = 1 g of HeliumArgon: 0,25 moles ×
= 1 g of HeliumArgon: 0,25 moles ×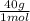 = 10 g of ArgonKrypton: 0,25 moles ×
= 10 g of ArgonKrypton: 0,25 moles ×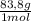 = 20,95 g of krypton Xenon: 0,25 moles ×
= 20,95 g of krypton Xenon: 0,25 moles × = 32,825 g of Xenon
= 32,825 g of Xenon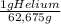 × 100 = 1,6%
× 100 = 1,6% = 64,775 g/mol
= 64,775 g/mol = M
= M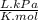
 ×
× 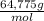 ×
× 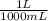 =
=

