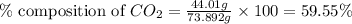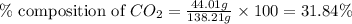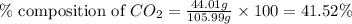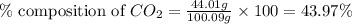A1.1000 gram carbonate sample chosen from li2co3, k2co3, na2co3 and caco3 was reacted with h2so4 and was found to lose 0.3497 gram of co2. (1) show the calculation of the % co2 in the unknown carbonate sample. (2) show the calculation of the % co2 in each of the carbonate compounds and identify the unknown carbonate from the list. atomic weights: c = 12.01, o = 16.00. mws: li2co3 = 73.892, k2co3 = 138.21, na2co3 = 105.99 and caco3 = 100.09

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
A1.1000 gram carbonate sample chosen from li2co3, k2co3, na2co3 and caco3 was reacted with h2so4 and...
Questions




Mathematics, 20.01.2021 19:30

Biology, 20.01.2021 19:30



History, 20.01.2021 19:30





English, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30

Mathematics, 20.01.2021 19:30

Health, 20.01.2021 19:30

English, 20.01.2021 19:30

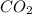 in unknown carbonate sample is 31.79 %
in unknown carbonate sample is 31.79 %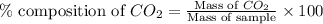 .....(1)
.....(1)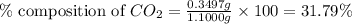
![[1\times 12.01)+(2\times 16.00)]=44.01g](/tpl/images/0237/8770/41a6a.png)