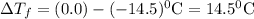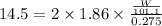Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at −14.5 ∘c? the freezing point for pure water is 0.0 ∘c and kf is equal to 1.86 ∘c/m. express your answer to three significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of wate...
Questions

Mathematics, 10.07.2019 15:00


Biology, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00


World Languages, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00

Spanish, 10.07.2019 15:00


Social Studies, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00




Spanish, 10.07.2019 15:00

Mathematics, 10.07.2019 15:00




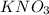 must be added
must be added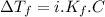
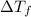 is depression in freezing point of solution, i is van't hoff factor (equal to number of ions produce from dissociation of 1 molecule of electrolyte) and C is molality of solutionMolar mass of
is depression in freezing point of solution, i is van't hoff factor (equal to number of ions produce from dissociation of 1 molecule of electrolyte) and C is molality of solutionMolar mass of 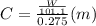 For
For 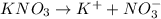 )Here
)Here