
Chemistry, 18.09.2019 01:00 emalvidrez5205
Consider the following reaction: chcl3(g) + cl2(g) → ccl4(g) + hcl(g) the initial rate of the reaction is measured at several different concentrations of the reactants with the following results: [chcl3] (m) [cl2] (m) initial rate (m/s) 0.010 0.010 0.0035 0.020 0.010 0.0069 0.020 0.020 0.0098 0.040 0.040 0.027 from the data, choose the correct rate law for the reaction. rate=k[chcl3][cl2]2 rate=k[chcl3][cl2]12 rate=k[chcl3]2[cl2] rate=k[chcl3]12[cl2]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Consider the following reaction: chcl3(g) + cl2(g) → ccl4(g) + hcl(g) the initial rate of the react...
Questions


Spanish, 17.12.2020 22:00

Mathematics, 17.12.2020 22:00

Mathematics, 17.12.2020 22:00

Spanish, 17.12.2020 22:00


English, 17.12.2020 22:00



Mathematics, 17.12.2020 22:00

Spanish, 17.12.2020 22:00



Mathematics, 17.12.2020 22:00

Biology, 17.12.2020 22:00

Chemistry, 17.12.2020 22:00



Mathematics, 17.12.2020 22:00

![\text{Rate}=k[CHCl_3][Cl_2]^{1/2}](/tpl/images/0238/0898/b5249.png)
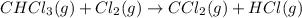
![\text{Rate}=k[CHCl_3]^a[Cl_2]^b](/tpl/images/0238/0898/f7b12.png)

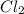
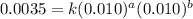 ....(1)
....(1)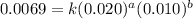 ....(2)
....(2)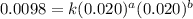 ....(3)
....(3)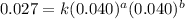 ....(4)
....(4)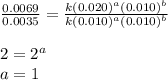
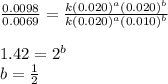
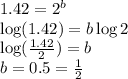
![\text{Rate}=k[CHCl_3]^1[Cl_2]^{1/2}](/tpl/images/0238/0898/0ea6b.png)


