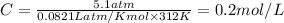The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant r. suppose the osmotic pressure of a certain solution is measured to be 5.1 atm at an absolute temperature of 312 k. write an equation that will let you calculate the molarity of this solution. your equation should contain only symbols. be sure you define each symbol other than r .

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Brad and his family like to go camping. they always take a gas lantern with them. the lantern can take fiuels but always puts out the same amount of light. brad has two different kinds of fuel for the lantern. how can he figure out which kind of fuel has more chemical energy that the lantern can turn into light? measure the brightness of the lantern using each kind of fuel by comparing it to the full moon. g measure the volume of the containers of fuel. measure how long the lantern stays lit on a fixed amount of fuel. measure the mass of each container of fuel using a balance scale. answer 12
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions




Mathematics, 16.04.2021 03:50




Chemistry, 16.04.2021 03:50



Physics, 16.04.2021 03:50

Social Studies, 16.04.2021 03:50


Mathematics, 16.04.2021 03:50

History, 16.04.2021 03:50

Mathematics, 16.04.2021 03:50




Mathematics, 16.04.2021 03:50

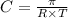

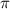 = osmotic pressure = 5.1 atm
= osmotic pressure = 5.1 atm